Bioanalytical and Pharmaceutical Research Laboratories
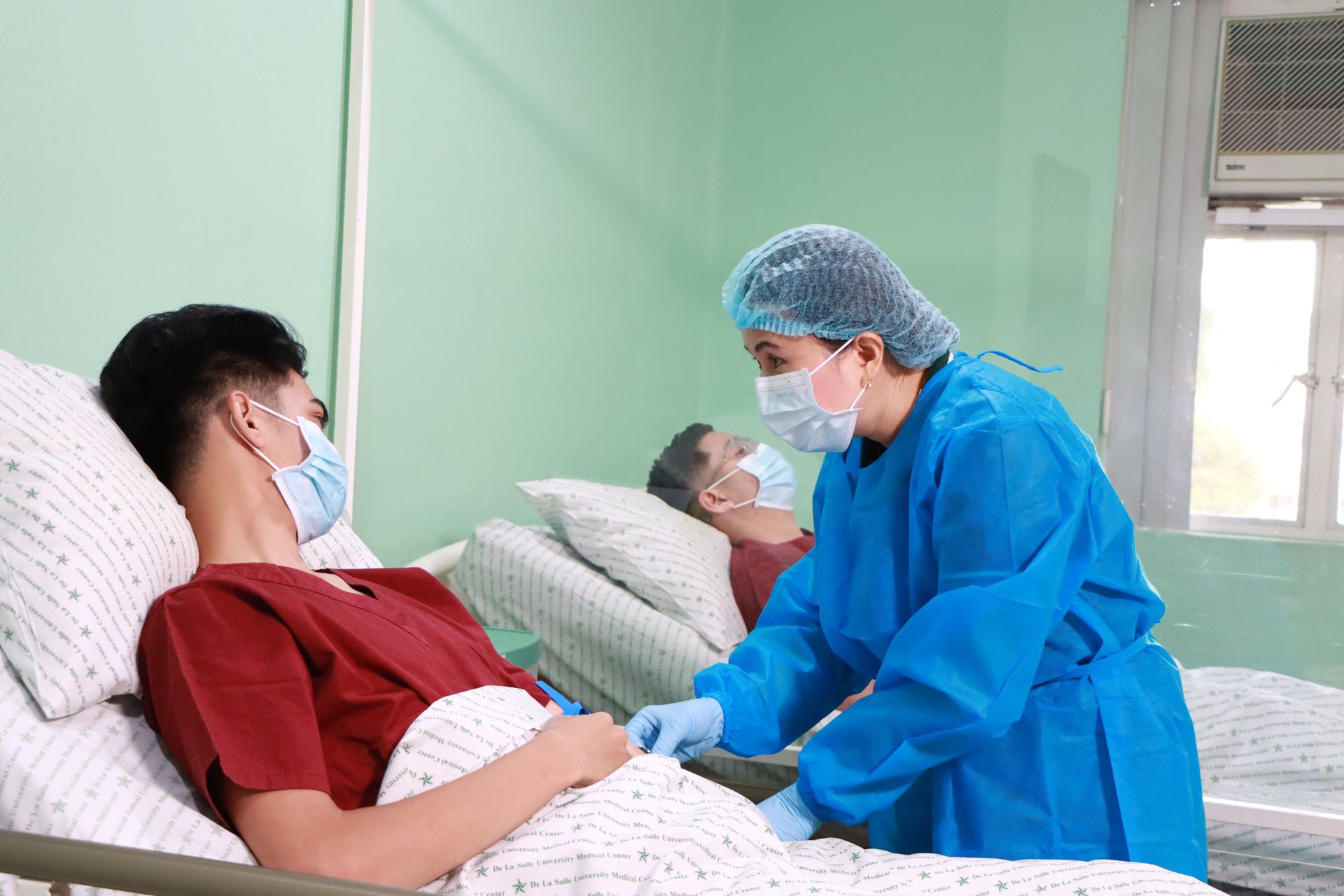
The Bioanalytical and Pharmaceutical Research Laboratories specializes in conducting rigorous assessments of bioavailability and bioequivalence (BA/BE) to ensure the safety and efficacy of generic medications. We operate an FDA-accredited clinical research unit with a 16-bed capacity, designed to provide comprehensive monitoring of participants. This facility supports overnight stays, continuous health assessments, and a controlled environment, ensuring precise data collection throughout the study.
SERVICES OFFERED

Bioavailability and Bioequivalence
(BA/BE) Testing
We provide single-dose and multiple-dose pharmacokinetic studies to evaluate the absorption, distribution, and elimination of drugs, ensuring their equivalence to innovator products.

Safety and Toxicity Monitoring
Our team conducts safety assessments for newly marketed drugs and their generic counterparts, ensuring compliance with regulatory standards.
First-in-Human (FIH) Studies
We offer pharmacokinetic evaluations in first-in-human trials to assess the safety, tolerability, and drug behavior in the body.

Therapeutic Drug Monitoring (TDM)
We provide therapeutic drug monitoring as part of BA/BE testing to optimize drug efficacy and safety.

FDA Consultation and Regulatory Support
Our expertise includes guiding clients through the FDA approval process, from consultation to final approval of BA/BE studies and other regulatory submissions.
Contact Us
Bioanalytical and Pharmaceutical Research Laboratories
Rm. 5100, Ground Floor, Lourdes E. Campos, MD Building
De La Salle Medical and Health Sciences Institute
Governor D. Mangubat Avenue, Zone IV, City of Dasmariñas, Cavite, Philippines
+63 (2) 8988-3100 (Manila)
+63 (46) 481-8000 (Cavite)
Local no.: 1386
PHONE
Monday to Friday
8:00 AM - 5:00 PM


